Wi-Fi ready
Ocean salinity
Salinity measurements are a fundamental part of any oceanographic research. Along with temperature, salinity determines the density of the water. Density drives local processes, which in turn affect global circulation and energy transfer in the ocean. This has broad implications, as energy transfer dictates both local and global climate. Salinity levels also have implications for biological processes such as algal blooms and other marine life, and affect applications like bathymetry and acoustic measurements.
Table of contents

What is ocean salinity and how do we measure it?
What are typical values for ocean salinity?
Ocean salinity is the ratio of grams of salt per kilogram (or litre) of water. This is a dimensionless unit and is typically expressed as parts per thousand (ppt). Ocean salinity may also be expressed in practical salinity units (PSU). About 90% of all dissolved ions in seawater are either sodium or chloride. Others include sulfate, magnesium, calcium, and potassium.
The average ocean salinity is just under 35ppt, and typical salinity values in the ocean range between 33ppt and 37ppt. However, lower values are found in enclosed seas at higher latitudes, such as the Baltic Sea; in coastal waters with increased river runoff; and in areas where precipitation is higher than evaporation. In contrast, higher salinity values are found where there is limited river runoff, high evaporation, and low precipitation, for example in the Mediterranean and the Red Sea.
How do CTDs measure conductivity in seawater?
Conductivity sensors all rely on the same general principle: an electrical current is induced in a small volume of seawater to measure its electrical resistivity, i.e. its ability to oppose an electric current. The resistivity of the seawater, expressed in ohms (Ω), is the reciprocal quantity to its electrical conductance, in Siemens (S), and is a measure of the seawater’s capacity to pass the electric current. Conductance can then be converted to seawater conductivity, in S/m, by scaling the measured conductance by the conductivity cell constant. Seawater conductivity is also commonly expressed in mS/cm.
Theoretically, the cell constant is defined as the ratio of the conductivity cell’s length to its cross-sectional area. However, this idealized definition relies on assumptions that break down when considering the practicalities of realizable conductivity cells. In practice, the cell constant of a conductivity cell is determined empirically in the laboratory, by calculating the ratio of the measured conductance to a known reference conductivity. The cell constant of the conductivity cell must be scaled carefully depending on the objective, as it directly impacts both the range and the resolution of the final conductivity measurements. In the case of seawater salinity, the conductivity cell is typically designed to have a cell constant that allows the sensors to capture a conductivity range of 0mS/cm and 85mS/cm, at a resolution of approximatively 0.001mS/cm.
What are the most common conductivity sensors used in CTDs?
Where conductivity cell technology differs across CTD manufacturers is in the technical approach taken to generate the electrical current in the sampled volume of seawater. The two most common approaches are inductive conductivity cell sensors and electrode-based sensors.
Inductive conductivity sensors
Inductive conductivity sensors, like the RBR conductivity cell, rely on Faraday’s law of induction. The conductivity cell is composed of a wire coiled around an annulus made of ferrite. A current is run through the wire, generating a magnetic field within the ferrite. The circular magnetic field in turn generates an electric field through the centre of the annulus, directly into the seawater. The conductivity sensor also includes a second “receiving” coil that reverses the process: the electric current that has travelled through the seawater is picked up by the receiving coil. The difference in the emitted and the received electrical fields gives a direct measurement of the seawater conductivity the electric field has travelled through. Laboratory calibration of the sensor provides information on the relationship between the measured current and the seawater conductivity, based on the cell’s constant, therefore yielding a precise measurement of seawater conductivity.
Advantages:
- Rugged sensor construction withstands rough handling and can be used in freezing conditions
- Naturally flushed, pump-free design uses relatively little power offering longer autonomous deployments on a variety of platforms
- Noise-free design will not affect passive acoustic monitoring
- Sensors are unaffected by trace amount of contaminants and able to measure within 20cm of the air-sea interface
Drawbacks:
- Sensors need to be calibrated in fully-integrated state in order to avoid proximity effects and obtain stated accuracy
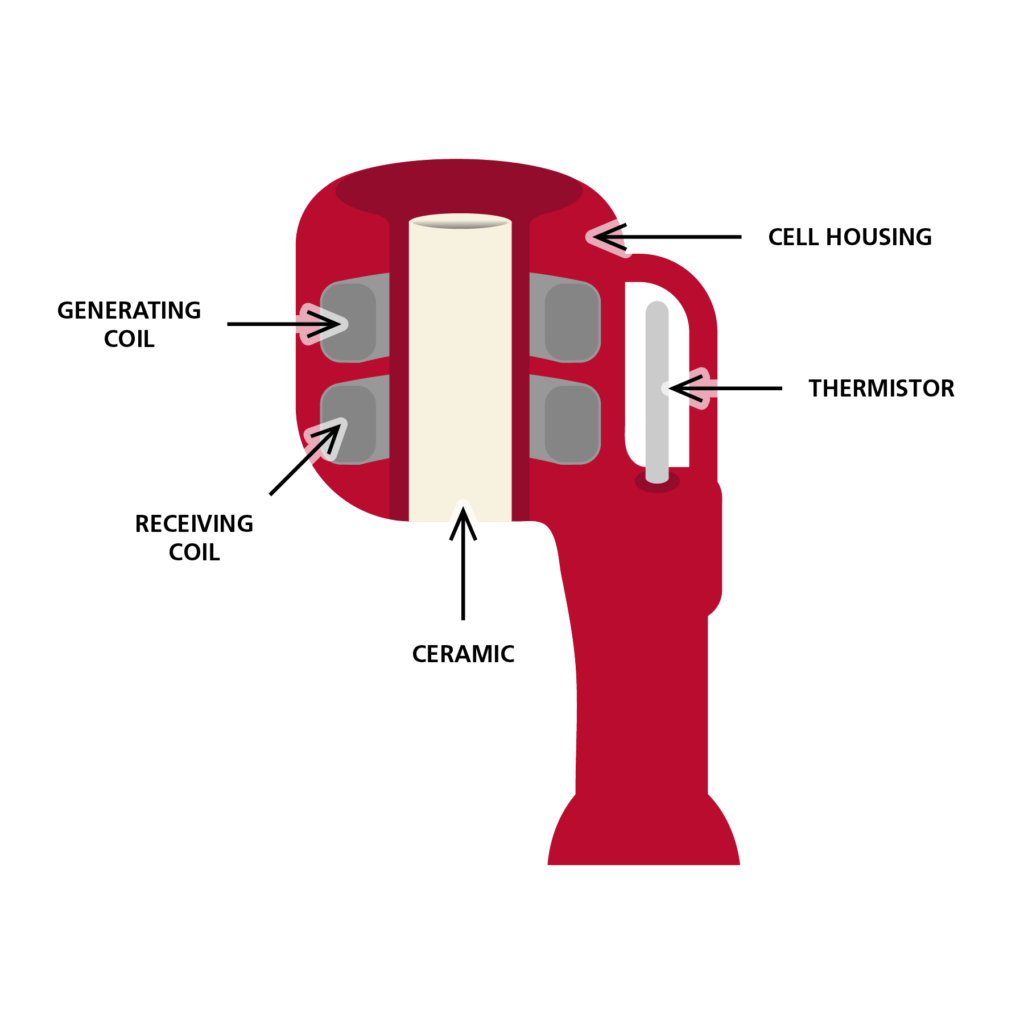
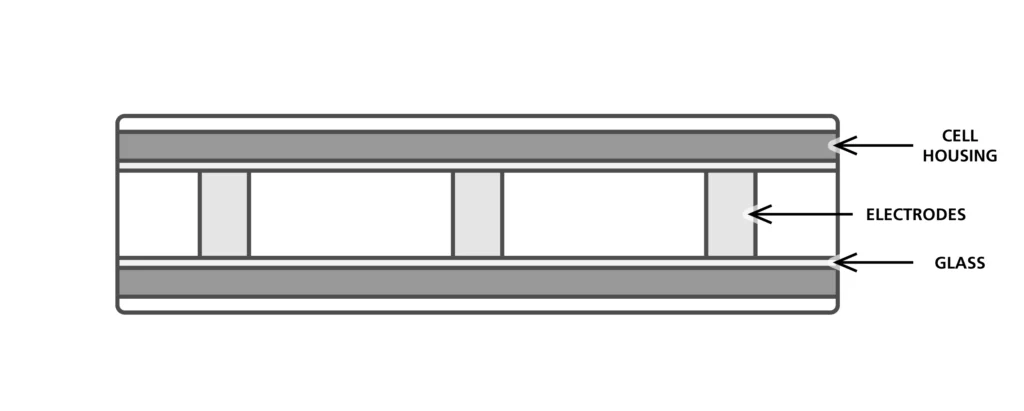
Electrode-based conductivity sensors
Electrode-based conductivity sensors rely on measuring seawater resistivity in a small volume of water contained in an insulating material, often borosilicate glass, in which three electrodes are located. The seawater’s conductivity is deduced from the measured resistivity between the electrodes.
Advantages:
- Fully-enclosed electromagnetic field is unaffected by nearby objects (such as antennas, sensor guards, or other instruments)
- Temperature and conductivity are measured on the same small water parcel
Drawbacks:
- Complex assembly is difficult to clean in the field and is not suitable for use in freezing conditions
- Pump requires power to operate which impacts the endurance of the instruments and/or the size of the required power supply
- Pump causes vibrations and noise which may affect sensitive acoustic or microstructure measurements
- Electrode surfaces can be easily fouled by surface contaminants, so pump must be stopped metres before reaching surface
How do we calculate salinity from CTD measurements?
Once conductivity is measured, it can be linked to the seawater salinity. The challenge is that conductivity does not only depend on seawater salinity, it is also a function of temperature and pressure. One must first remove the impact of temperature and pressure on the measured conductivity. This is achieved using a non-linear equation that is considered the international standard for computing salinity from conductivity. This is referred to as the Practical Salinity Scale 1978, or more commonly as PSS-78.
The PSS-78 scale defines a reference seawater composition with a conductivity of 42.9mS/cm at a temperature of 15°C and a pressure of 1atm. The salinity of a seawater sample is then calculated relative to this reference composition using a set of empirical relationships and correction factors to account for the impact of temperature and pressure. The resulting value is expressed in practical salinity units, which are defined as the ratio of the measured conductivity of the sample to the conductivity of the reference seawater composition, and is therefore dimensionless.
For reference, a change in conductivity of 1uS/cm corresponds to a change in salinity of approximately 1mPSU. Similarly, a change in temperature of 1m°C corresponds to a change in salinity of approximately 1mPSU.
The PSS-78 scale is widely used in oceanography and other fields as a standard way of expressing the salinity of seawater. While it is considered to be accurate and reliable, it is based on empirical relationships and may not be applicable to seawater compositions that differ significantly from the reference seawater composition.
In 2010, the Intergovernmental Oceanographic Commission (IOC), International Association for the Physical Sciences of the Oceans (IAPSO), and the Scientific Committee on Oceanic Research (SCOR) adopted a new set of standard equations to compute thermodynamic properties of seawater (TEOS-10). In the process, the concept of “Absolute Salinity” was introduced: a true mass fraction, expressed in g/kg, that captures the different chemical composition of real seawater throughout the ocean. This concept is now widely used as it more accurately reflects spatial variations.
Dive deeper

How to select the right CTD for your application
Different CTDs excel in different applications, and with RBR’s wide range of available CTDs.

How to select the right CTD [Webinar]
Learn about technical requirements and considerations for specific applications and what CTD options are best for each one.

All about speed of sound in water - what it is, how it's measured, and why we care
Sound-speed in water is a quantification of how fast sounds travel in water.

A proven standard in CTD sensor technology: RBR’s inductive conductivity cell
There are many tools to measure the physical properties of the ocean, yet few are as universal as the CTD.
Featured instrument
RBRconcerto³ C.T.D
Uniquely designed to determine salinity by measuring the conductivity and temperature of water. Conductivity measurements are performed using a rugged inductive cell that can be frozen into ice. Equipped with a pressure channel, the RBRconcerto³ C.T.D can also derive depth, density anomaly, and speed of sound.

Explore popular CTD models
Read more user stories

The SUNRISE Project: Using RBR instruments to unravel mixing in the Gulf of Mexico
The coastal ocean is a dynamic and productive environment, critical for the success of fisheries, tourism, and local economies.

Pushing what's possible: High resolution estuarine sampling using rapid vertical profiling
Everything Dr. Rocky Geyer studies boils down to a deep love of nature and physics.

FjordPhyto: Citizen science in the Antarctic
Air and ocean temperatures have notably increased in the Antarctic since the 1950s.

Monitoring sound in the Salish Sea: how scientists are trying to understand the decline of Southern Resident Killer Whales
Studying the home of the Southern Resident Killer Whales – a declining species of whale located in the eastern Pacific.